Sunday, May 1, 2016
That's All Folks! (But Not Really)
Though my daily routine at my internship did not lend itself very well to being described on a blog as it was largely reading journals, taking down data, and analyzing the said data I am glad to have been able to share what I learned along the way with those of you on my blog. I would like to thank all of the BASIS faculty members (as well as my external advisor) who helped us along the way with our projects and I would also like to congratulate all of the other seniors finishing up their projects. This has been an incredible learning experience, not just on my particular topic, but on what research actually entails, how to present my findings in a way that's meaningful, and also how to adhere to a schedule (still working on this last point). All of the projects being done here at BASIS are amazing and I am excited to see everyone present this coming week. As for what will happen on the blog, I plan on posting my research materials and some data on the second page and a copy of my presentation (for those who can't see it for some reason want to see it again). There may also be some surprise posts in the future, so if you're interested stay posted.
Possible Drug Treatment for BK Virus Nephropathy (Part 2)
The final and most widely used agent used as a therapy against the BK virus is Leflunomide. Leflunomide is an immunosuppressive agent which is known to block dihydroorotate dehydrogenase, tyrosine kinase, and pyrimidine synthesis. It is licensed for treatment of rheumatoid arthritis and has been used in combination with immunosuppression reduction in the treatment of BK virus-associated diseases. It has modest in vitro activity, however, much like some of the other drugs discussed previously in vivo data is both scarce and sometimes conflicting. What is known is that it can serve the dual purpose of both an antiviral as well as an immunosuppressant in high enough doses (higher than those generally given for rheumatoid arthritis). The other main benefit of Leflunomide which makes it one of the most promising candidates as a therapy to fight the BK virus is its oral bioavailability and its relatively low nephrotoxicity. The factor which limits its dosage is damage to the liver (the same also being true for Dayquil and cough syrup).
Thursday, April 28, 2016
Hypertrophy of the Kidney (part 2) With Pictures!
 In a 2009 study, compensatory renal enlargement was assessed in 19 adult patients who either had a nephrectomy (the removal of a nephron, 17 cases) or developed a functionless kidney following obstruction (two cases). Hypertrophy of the healthy kidney was quantified by comparing renal size on urography (X-ray after the injection of radiographic contrast material) before and after removal or destruction of the diseased kidney. 40% of the patients within the study showed compensatory enlargement, including (most surprisingly) two patients in their sixties. The average increase in length was 3% and the maximum increase in length was 9%. The results of the study showed that compensatory enlargement occurs during both adulthood and childhood. This contrasts with the initial belief that compensatory enlargement occurred only during childhood. The increase in kidney size is, however, significantly larger if the destruction or removal occurred earlier in life. The study also showed that the presence of a hypertrophied adult kidney over 17 cm in length usually indicates that the contralateral renal disease was present in childhood (affecting both the destroyed kidney as well as the previously healthy one). In summary, the kidney has a mechanism (though it is limited) to be able to compensate for both loss of nephrons, as well as a reduction in nephron efficiency, regardless of the cause. Bringing this back around towards my focus in this project, it shows that even if the tubular atrophy and interstitial fibrosis caused by BK virus nephropathy (see post on BK Virus Nephropathy) can never be repaired, by stopping or slowing the progression of the disease, the kidney can take steps to bring itself closer to its original functionality. The irreversibility of the damage still highlights the need for close monitoring of transplant patients’ grafts as well as preventatively creating the most balanced immunosuppressive regimen for the patient.
In a 2009 study, compensatory renal enlargement was assessed in 19 adult patients who either had a nephrectomy (the removal of a nephron, 17 cases) or developed a functionless kidney following obstruction (two cases). Hypertrophy of the healthy kidney was quantified by comparing renal size on urography (X-ray after the injection of radiographic contrast material) before and after removal or destruction of the diseased kidney. 40% of the patients within the study showed compensatory enlargement, including (most surprisingly) two patients in their sixties. The average increase in length was 3% and the maximum increase in length was 9%. The results of the study showed that compensatory enlargement occurs during both adulthood and childhood. This contrasts with the initial belief that compensatory enlargement occurred only during childhood. The increase in kidney size is, however, significantly larger if the destruction or removal occurred earlier in life. The study also showed that the presence of a hypertrophied adult kidney over 17 cm in length usually indicates that the contralateral renal disease was present in childhood (affecting both the destroyed kidney as well as the previously healthy one). In summary, the kidney has a mechanism (though it is limited) to be able to compensate for both loss of nephrons, as well as a reduction in nephron efficiency, regardless of the cause. Bringing this back around towards my focus in this project, it shows that even if the tubular atrophy and interstitial fibrosis caused by BK virus nephropathy (see post on BK Virus Nephropathy) can never be repaired, by stopping or slowing the progression of the disease, the kidney can take steps to bring itself closer to its original functionality. The irreversibility of the damage still highlights the need for close monitoring of transplant patients’ grafts as well as preventatively creating the most balanced immunosuppressive regimen for the patient.Hypertrophy of the Kidney (Part 1)
When most people discuss plasticity it's most often with respect to the brain. Neuroplasticity is the trait of the brain where it can change its functional structures to fit a new need by forming new neural connections. An example would be how after a brain injury during which damage was sustained to the parts of the brain which handle speech, the tasks of the damaged portions can be taken up by other regions of the brain. It is even possible that functionality will return to normal or at least improve. The kidney, unfortunately, does not behave the same way.
Kidney damage can rarely ever be reversed and the progression of chronic kidney disease can really only ever be slowed down, not stopped. In addition to this, at around the age of 40 the kidney begins to lose functional nephrons (they become obsolescent nephrons), renal plasma (due to the absorption of obsolescent nephrons), and have an overall decrease in the glomerular filtration rate (GFR). While the list of things that can harm or reduce its functionality is incredibly expansive the kidney does, however, have a mechanism for dealing managing those factors that is somewhat similar to plasticity in the brain.
The reason people can live normal lives past the age of 50 is because of compensatory hypertrophy. Hypertrophy is defined as the increase in the volume of an organ or tissue due to the enlargement of its component cells. With respect to the kidney, this means that nephrons in a sense swell up in order to be able to filter more more blood, thus compensating (for the most part) for nephron reduction due to old age. This process is seen to occur most strikingly in patients who have lost all (or nearly all) function in one of their kidneys.
Wednesday, April 27, 2016
Palette Cleanser: 18 of the Most Interesting Kidney Facts I Could Find
In the midst of many blogposts regarding many of the more technical aspects of my project, I wanted to include at least one post showing some fun characteristics of the kidney.
1. Each individual kidney consists of at least 1 million and up to 2 million nephrons.
Nephrons are nothing are the very tiny filters shown above which eliminate waste materials.
2. Within a single hour, kidneys receive around 120 pints of blood.
3. Despite accounting for only 0.5% of body weight almost 25% of the blood pumped by the heart goes to the kidneys.
4. Once a person reaches the age of 40, the number of functional nephrons present in each kidney start falling at a rate of 1% a year.
5. Despite the decline in the number of functional nephrons in kidneys after the age of 40, kidneys continue to function normally because the remaining nephrons enlarge to handle the increased flow (called hypertrophy).
6. If the nephrons in both kidneys are taken out and placed end to end horizontally, they will cover a distance of 16 kilometers.
7. If one kidney is taken away and the functional capacity of the other kidney is reduced to just 75%, it can still sustain life!
8. Another essential function kidneys perform is maintaining a constant amount of fluid in the body and so the entire blood supply in the body gets filtered around 400 times in a day through the kidneys.
9. If the blood pressure in kidneys fall, they start sending out signals to the rest of the body. As a result of these signals vasoconstriction occurs and pressure can return to normal.
10. Kidneys can also detect if the oxygen content of the blood falls. Once the kidneys sense a lack of oxygen, they secrete a hormone which triggers an increased production of red blood cells.
11. Some children are born with only one kidney. For them, the single kidney eventually grows to the extent where its weight is equal to the combined weight of two kidneys.
12. Excessive milk can cause kidney stones (my greatest fear).
13. Malfunctioning kidneys can lead to the development of anemia.
14. High blood pressure and diabetes can both lead to failure of kidneys (See my post about risk factors during transplant!)
15. The first ever kidney transplant was conducted by Yuri Voronoy, a Russian surgeon in 1933. The transplant failed.
16. The first ever successful kidney transplant was conducted by Dr. Joseph E. Murray in December 1954. The transplant was between two identical twins and took place in at Peter Bent Brigham Hospital, MA.
17. Nearly 700 million people globally (which nearly 10% of global adult population) suffer from some kind of kidney problem/damage. This leads or millions of premature deaths both from kidney disease as well as the related induced cardiovascular diseases.
18. Nearly 1.5 million globally go through kidney transplant or kidney dialysis (why dealing with BK Virus related issues is significant!)
Tuesday, April 26, 2016
Possible Drug Treatment for BK Virus Nephropathy (Part 1)
As I have stated in previous posts, the only therapy for BK nephropathy is immunosuppressive reduction, however, this also puts the patient at risk for graft rejection.
At the moment there are several non FDA approved drugs which show promise in counteracting the BK virus. So far they are approved for treatment of several DNA viruses such as Herpes simplex, CMV, Hepatitis. All of these drugs act by inhibiting DNA polymerases effectively halting viral replication.
Each of the following paragraphs will be brief descriptions the specific drugs that may in the future be used for treatment.
Cidofovir:
Cidofovir stops viral replication by inhibiting the viral DNA polymerase. Cidofovir is currently approved for the treatment of CMV-induced retinitis in HIV-infected patients. The CMV virus is very similar to the BK virus in that it is widespread among the population and those infected remain largely asymptomatic. This drug treats inflammation of the retina caused by CMV when the patient's immune system is weakened by HIV. So far Cidofovir has shown in vitro (outside of the body; petri dishes) activity against the BK virus; however, there are conflicting reports of in vivo activity. Cidofovir has so far been used to treat patients with both BK-related hemorrhagic cystitis and CMV infection. It was found to reduce both CMV replication and the level of BK viruria, and resulted in clinical improvement. Low dose Cidofovir treatment has been used in bone marrow transplants with positive clinical outcomes and decreased viruria and viremia in 84% and 47% of the patients respectively. There are, however, also reports claiming the deleterious effects of reduced renal function and increased viral load in Cidofovir-treated BKVAN patients. Cidofovir also has limited treatment potential in renal transplant patients due to its nephrotoxicity as well as its limited oral bioavailability. The variable and the conflicting results of Cidofovir treatment show the need for randomized clinical drug trials.
CMX001:
CMX001 is a slightly better alternative to Cidofovir in that it is orally delivered and has reduced nephrotoxicity. It is also able to inhibit BK Virus replication much more rapidly and with a longer-lasting effect. This compound was tested in renal transplant and BMT recipients but the results of this clinical study were not yet available at the time this review was written.
As a fun fact this compounds full name of all individual elements in this compound are
phosphoric acid; [[(S)-2-(4-amino-2-oxo-1(2H)-pyrimidinyl)-1-(hydroxymethyl) ethoxy]methyl]mono[3-(hexadecyloxy)propyl] ester; hexadecyloxypropyl cidofovir
Tuesday, April 12, 2016
Data and Statistics for the Previous Post
My previous post gave the final result of the study, but not any of the hard numbers that give it credence. I hope to address that here.
The study (conducted over a 1 year interval) showed that by 3 months, the rates of viremia and viruria in both the Tac-arm and the CsA-arm had increased by nearly the same amount, 10% and 14% with CsA having the larger percentage. At 6 months something interesting occurred the two rates diverging and reversing, with the Tac-arm having 16.3% Viremia and CsA having 10.6%. This trend was shown continuing at the 12 month mark (Tac-12.1%, CsA-4.8%).
This therefore shows the significantly increased risk of developing BK nephropathy when given high potency agents. This should not, however, be interpreted to mean that CsA’s benefits automatically outweigh its costs. Weaker immunosuppression runs the higher risk of having the body inflict damage to the graft. What should be taken away from this study is the importance of balance. Finding the balance between the risk of developing BK related issues and the risk of an immune response for each individual person remains the ultimate goal for maximizing kidney graft longevity and thus bettering the life of the patient.
Attached above is a picture showing the graphed trends (%) for BK Viruria (left) and BK Viremia (right) over the one year interval with Cyclosporine being the solid point line and Tacrolimus being hollow.
How Immunosuppressants Have Evolved
It has been requested that I do a post on the differences between early immunosuppressive agents and the ones that we currently use. One way to compare these differences in terms that most people are familiar with is likening the immunosuppressive agents to antibiotics. In the same way that separate classes of antibiotics disrupt bacteria multiplication through different mechanisms, these agents each intervene with specific functions of differing cells within the immune response. One of my earlier posts shows how several induction agents either prevented the antigen presenting cell from communicating with the T-cell or directly interfere with the response of the T-cell itself. Early exploration and differentiation of these agents was mainly focused on finding different mechanisms of action, searching for one which had the fewest number of collateral effects. Relating back to the my timeline of maintenance and induction agents, the main difference between earlier agents and ones that are used now lies in side effects (generally lesser with newer medication) and potency (how much it curbs immune response per a given dosage).
A higher strength agent is not, however, always the most desirable to use. One particular article which compares the the incidence of BK viremia (BK Virus presence in the bloodstream) and BK viruria (presence in the urine, which implies a strong presence in the kidney) of two different maintenance agents shows the risk of using the stronger agent. The study included 629 patients of various backgrounds who were randomly assigned a immunosuppressive regimen of either Tacrolimus (the most widely used and more powerful agent) or Cyclosporine (relatively weaker). Though the study did not go further and explore whether one drug has a higher rate of developing BK nephropathy (the actual damage to the kidney) it can be safely assumed that higher rates of viremia correlate with higher rates of nephropathy. The study showed significant difference in the rates of BK Viremia between the two agents.
Tuesday, March 29, 2016
What Exactly Happens on the Cellular Level (With Fun Pictures!)
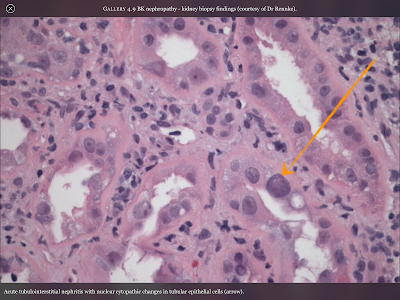
Pictured directly to the left is a cross-section of the the inside of a renal medulla (where filtration occurs). The small purple colored dots are the nuclei of the kidney’s epithelial cells which make up structures such as the walls of capillaries and microtubules. Because this is cross section the actual tubule is white space surrounded by a ring of cells, like looking at a pipe through the hole. The orange arrow is pointing at an epithelial cell that has been altered by the cytopathic effects of the BK Virus. It becomes enlarged and as you can see the tubule will begin to be closed up by the malformed cells, as well as the resulting scar-tissue.
 In the picture to the right, cells with a BK nuclear intrusion are highlighted using SV40 immunostaining. SV40 (Simian virus 40) is a virus in the same family as the BK (polyoma viruses) that can infect both humans and simians alike. Using it to stain locations of BK intrusion is another example of how we rely on research animals to be better able to help and understand ourselves, much like with rATG and hATG (see rATG post).
In the picture to the right, cells with a BK nuclear intrusion are highlighted using SV40 immunostaining. SV40 (Simian virus 40) is a virus in the same family as the BK (polyoma viruses) that can infect both humans and simians alike. Using it to stain locations of BK intrusion is another example of how we rely on research animals to be better able to help and understand ourselves, much like with rATG and hATG (see rATG post).
The final image shows fluorescent tagged immunoglobulin which is concentrated around areas of what is thought to be BK virus cytopathic manifestation.
BK Virus Nephropathy: History, Facts, and the Results of my Research on the Manifestations of the BK Virus (Part 2)
As mentioned in the previous post, it is for the most part unknown if a patient has progressed to nephropathy until the later stages of the disease. Because of this it is important to analyze a person’s risk for BKVAN by assessing them based on a number factors. Accurately predicting if someone is more likely to develop the disease preemptively is incredibly useful, as lowering the immunosuppressive load before onset is far more effective in BK Virus replication than doing so retroactively.
The risk factors can be broken down into three main categories: donor factors, transplant factors, and recipient factors. Donor factors are risk factors that relate to the condition of the person who is donating the kidney. These include BKV Seropositive status (whether the BK virus is in the blood of the donor) and absence of HLA C7 (important marker protein). The transplant factors mostly relate to the interactions between the donated kidney and the immune system of the recipient. These include whether or not (or to what extent) the kidney (or the surrounding tissues) was damaged during transplant, ABO incompatibility (differing blood types), and whether or not the recipient has had a rejection before. Typically if the transplant recipient has had previous transplants the immune system is already prepared to attack the graft and so rejection is more likely, but how this correlates to the recurrence of BKVAN is unknown. The final category, recipient factors, relate to various aspects of the host's body. They include things such as age, BK seronegative status, presence of diabetes, absence of HLA 7 and the need for immunosuppression.
After having compiled data for 237 HLA mismatched patients and 72 ABO-Incompatible patients between 1998 and 2010 I found a 6.1% and 17.8% prevalence of BKVAN respectively (p=0.08, a=0.05). Thus it can be seen beyond a reasonable doubt that the risk for developing it is severely higher when the graft and the recipient are not of the same blood type. While this correlation is important in and of itself, the reasons behind it are even more so. Because of the presence of such severely distinct markers on the graft, the recipient's immune system launches a much stronger attack on the new kidney thus necessitating a larger immunosuppressive load. The correlation between higher dosages of immunosuppressants and an increased risk for BKVAN gives credence to my initial hypotheses (see the first blogpost).
BK Virus Nephropathy: History, Facts, and the Results of my Research on the Manifestations of the BK Virus (Part 1)
BK Virus Nephropathy (BKVAN) is the clinical term for the condition in which the BK virus is actively harming the recipient's kidney graft. About 10% of renal transplant recipients will end up progressing from BK Viremia to BK Nephropathy. A plot of the data points of my collected data shows a slight upward increase in this number over the years 2000 to the 2014 ultimately leading to the most recent 10% statistic. A significant difference is found between the rates of incidence in first time and second transplant patients, with multi-transplant recipients being more at risk for nephropathy.
Though it is relatively easy to now detect whether or not the virus is in the bloodstream, it is much harder to see whether or not the viral infection has progressed to more severe stages. Renal biopsy (the sampling of some of the kidney tissue and then viewing it on a slide), remains the gold standard for recognizing BKVAN, however, many advancements in analyzing a hosts risk factors can lead to catching the disease in its earlier stages.
After a person has first come into contact with the virus (about 90% by the age of 23) it enters a state of non-replicative asymptomatic infection called “latency”. The very small virus particle embeds itself in the epithelial and urothelial cells in an immunocompetent host. Once an increased immunosuppressive load shifts the balance of immune system cells and virus particles towards the virus it begins to replicate and the graft goes through the three stages of BKVAN.
Stage A is where the virus starts to produce structural changes in the cells that make up the epithelial walls of the kidney. Unfortunately, it is hard for even a kidney biopsy conclusively determine that nephropathy is occurring at this early stage, often going undetected until it progresses to stage B. Stage B consists of tubulointerstitial inflammation; the swelling of the capillaries used throughout the kidney to carry and filter blood. Stage C is tubular atrophy and interstitial fibrosis which severely reduces the kidneys functionality and fills it with scar tissue.
Between 30 to 60% of BKVAN cases progress to Stage C.
Sunday, March 27, 2016
What It Is and How It’s Made? The Synthesis of Rabbit ATG (rATG)
Used in over 55% of transplant cases rATG is by far the most commonly used. When most people are asked to imagine how it is synthesized most would say by a process of mixing solutions, enzymes, and proteins in a lab. This induction agent, however, is actually one two such agents made by immunizing another species to our white blood cells. Serums from over 26,000 (2012) immunized rabbits are pooled to ensure batch to batch consistency, an incredible effort on the part of both rabbits and humans.
The first report of immunizing an animal of one species against the immune cells of another species (mouse lymphocytes) was by Russian zoologist Ilya Metchnikoff. His trial (in 1899) involved injecting cells taken from mouse lymph nodes into a guinea pig. This guinea pig was then given time to develop antibodies specific to the immune cells of the mouse. A serum (ATG) was crafted from the blood of the guinea pig and then reinjected into other healthy mice. Metchnikoff observed a drop in the T cell count of the mice, and thus made an incredible breakthrough in the new field of immunology (and would later win a nobel prize for his work).
Our process for synthesizing rATG nowadays is still very similar to Metchnikoff’s. The notable difference is that instead of mice we immunize the rabbits to our own T cells. Rabbits are mainly utilized because of their ability to reproduce very quickly. The downside, that much like a blood donation for humans, they can only give so much within a given time. It is for this reason that sometimes equine ATG is also used, with a horse being able to give much more per donation. rATG and hATG are functionally identical and so they are chosen based on current price and availability.
It’s incredible to think that in order to keep one person’s organ alive and functional within another we appropriated the immune system response of another species.
Timeline for Important Immunosuppressants
As Mrs. Q has stated in a comment on an earlier post, the field of medicine has advanced rapidly in the past several decades and as new technologies and techniques were being discovered and perfected the margin for error has slowly decreased until it reached the accuracy we have today. The first truly successful kidney transplant didn’t occur until 1954 and was done between two identical twins, thus no immunosuppressive treatment was given or needed. Between 1954 and the present, many other kidney transplants were necessary, but early on, not many were attempted as the outcome would most surely be acute rejection unless the graft came from an identical twin (even then, mutations have a chance of affecting the expression of protein markers). It wasn’t until 1957, that the first widely used induction therapy agent Imuran, was used. With it came an incredible amount of side effects, the most severe being an increased chance of developing T cell cancer. From then until 1971, we did not know that the BK virus even existed, nor did we know of its detrimental effects on kidney grafts. Reliable detection would not come until 1983 and wouldn’t be perfected for another few years after this date. Because of this data from the before 1985 is very unreliable.
Another important factor which affects the reliability of the data was the immunosuppressive agents available and how widespread their usage was. It is for this reason that I have included at the end of this post a timeline with the year that important induction and maintenance agents were first synthesized, and for many when their usage became widespread.
eATG-- started in 1970, fully established by 1980 (Equine Anti-thymocyte Globulin)
rATG-- started 1980, fully established by 1990 (Rabbit Anti-thymocyte Globulin)
OKT 3-- 1985
Campath-- 2000
Basiliximab--1998
Daclizumab--1997
Imuran-- 1957
Celcept-- 1995
Cyclosporine-- 1983
Prograf-- 1994
Sunday, March 20, 2016
The Curious Case of the Regulatory T-Cell, Part 2
This T-Cell is another example of the body’s many self-check mechanisms which prevent excessive immune reactions, and stop an immune response once the invading organisms have been successfully eliminated. These cells are not naive T-Cells and therefore particular to only one antigen and work to stop NK cell and efferent T-Cells that are still attacking that antigen. This cell went unnoticed for a long time and is still hard to study today because it expresses nearly identical protein markers as the efferent T-Cells (the ones responsible for attacking antigens). Eventually after being isolated, it was noticed that an increase in the number of regulatory T-cells differentiated with respect to the markers on the graft found in the graft’s microenvironment leads to the formation of a protective barrier, offering localized antigen specific immunosuppression (the holy grail of transplantation). Another interesting relationship is how regulatory T-Cells interact with cancer. Cancer can be defined as a failure of the immune system to eliminate a cell which has suffered some kind of damage and now continues to replicate unchecked. One of the main reasons that the body will often not attack the tumor is because it expresses the same protein markers as other healthy cells in the body and so the body thinks that it is attacking itself. It is now thought that regulatory T-cells, in addition to stopping autoimmune disorders, also plays a critical role in protecting the cancer. It was noticed that if there was a high concentration of these types of cells in the cancer microenvironment it usually meant that the cancer prognosis was very poor.
Research into these cells is still very new and a lot about how they function and how we can control their replication is still unknown. They may, however, prove to be very helpful in both finding a cure for cancer (or at least a way to diminish the effect of it) as well as opening the way for natural antigen specific immunosuppression.
The Curious Case of the Regulatory T-Cell, Part 1
Though BK nephropathy is a common risk of being on an immunosuppression regimen after transplantation (about 80% of the population is already infected with it) another major but less talked about risk is developing cancer. This is many times overlooked when one thinks of the negative effects of having an organ transplant, but the reality is that in order to keep a graft alive it is necessary to suppress the system in our bodies which fights cancer. However, good things can come from such morbid side effects as it is possible that we may have a found a potential way to both help increase the chance of graft survival and to reduce the malignancy of many cancers. Unfortunately, fighting cancer with this regimen may decrease the life expectancy of a graft, while supporting the graft may make it much harder to fight the cancer. The key to both treatments lies in how the elusive regulatory T-Cell functions.
Thursday, March 17, 2016
Immunosuppression, Maintenance Therapy
 You probably could have guessed that this was coming after the last post. Even though induction therapy plays a large role in reducing the number of acute rejections, when trying to minimize the risk of long term rejection, maintenance therapy is key. Maintenance therapy is the immunosuppressant regimen that the patient will be on for the rest of their lives. A universal limitation on all immunosuppressive drugs is that they are non-antigen specific, and so because this regimen will affect the whole of the patient’s immune response a long period of time, patients must first be assessed based on their immunological risk before any kind of treatment can be started. Patients who have had previous transplants, are young, are African-American, or have an aggressive autoimmune disorder are at a higher risk for rejection and so are typically given higher dosages or more potent maintenance agents. The elderly, first transplant patients, and those without an autoimmune disease are generally low-risk. The immune system naturally breaking down with age is thought to be the reason older patients need less suppression, however, the flip-side of this is that older transplant patients have a longer recovery from the operation. Maintenance immunosuppression agents are usually used in conjunction with one another, especially when their effective mechanisms complement each other. The three main drugs are Calcineurin Inhibitors (CNI), mTOR Inhibitors and other anti-proliferative agents, and just like in induction therapy all of these are usually supplemented with steroids.
You probably could have guessed that this was coming after the last post. Even though induction therapy plays a large role in reducing the number of acute rejections, when trying to minimize the risk of long term rejection, maintenance therapy is key. Maintenance therapy is the immunosuppressant regimen that the patient will be on for the rest of their lives. A universal limitation on all immunosuppressive drugs is that they are non-antigen specific, and so because this regimen will affect the whole of the patient’s immune response a long period of time, patients must first be assessed based on their immunological risk before any kind of treatment can be started. Patients who have had previous transplants, are young, are African-American, or have an aggressive autoimmune disorder are at a higher risk for rejection and so are typically given higher dosages or more potent maintenance agents. The elderly, first transplant patients, and those without an autoimmune disease are generally low-risk. The immune system naturally breaking down with age is thought to be the reason older patients need less suppression, however, the flip-side of this is that older transplant patients have a longer recovery from the operation. Maintenance immunosuppression agents are usually used in conjunction with one another, especially when their effective mechanisms complement each other. The three main drugs are Calcineurin Inhibitors (CNI), mTOR Inhibitors and other anti-proliferative agents, and just like in induction therapy all of these are usually supplemented with steroids.Immunosuppression, Induction Therapy
Now that we have an understanding of how the immune system works, I will begin to discuss the main therapies used to prevent it from doing its job. Medicines used very soon after the surgery to suppress the initial immune response fall into the category of induction therapy. Those used to prevent rejection in the long term fall into the category of maintenance therapy. The goal of induction therapy is reduce the risk of acute rejection of the graft and as of 2015 80% of kidney transplant centers in the US use induction agents as part of their immunosuppression protocols. Induction agents are generally used because of their ability to affect several of the most critically important cells (T-Cells and B-Cells) and to quickly and severely reduce their effectiveness. It is for this reason that doctors will often put off a patient’s transplant even if they have a minor infection. This first wave of immunosuppressants can make even a small infection proliferate which may ultimately lead to death. The most commonly used agents are rATG (which is actually synthesized from rabbits!), Basiliximab, and Alemtuzumab. Each stops the naive T-Cell (T-Cell before it is presented with a particular antigen to attack) from becoming active in different ways, but one thing they all have in common is that they do not interfere with the patient’s recovery from the surgery. The most common maintenance agents (CNIs) have the side effect of constricting the blood vessels, which can prolong the amount of time before a wound (such as surgical cuts) heals. Alongside the induction agents transplant patients are given high-doses of steroids. The agents are generally taken only once, immediately after the procedure, however, the medicine has a long half-life (time it takes for it for half the medicine to be filtered out) and may stay in the body, working, for several weeks. Below is a picture of which part in T-Cell differentiation each medicine affects (I thought it looked cool).
Wednesday, March 16, 2016
General Immune System
 |
| The Two Categories |
Wednesday, March 9, 2016
Are things getting worse or are we just now discovering how bad they were? One of the most difficult parts of analyzing apparent trends in the incidence of BK viremia is attributing it to the proper source. I in particular am now looking at the specific times after which new immunosuppressive agents began to be used more regularly and seeing if the graphed incidence rates I have compiled thus far show any significant changes at or around those times. One thing that may, however, be a confounding variable for the overall trend is the many advances we have made in virus detection over the past three decades. This could potentially mean that many graft losses in the past may have misattributed to causes other than BK viremia simply due to the uncertainty then found in detection techniques. The main way to detect polyomaviruses in general is the Urinary EM, to check for the BK virus specifically, however, Polymerase Chain Reactions are used. This is a not a new process (being first done successfully in 1983), but like with most things we’ve polished and and refined it, slowly eliminating as much room for error as possible. Due to the time it took to figure out the optimal incubator times, temperatures and which ions disrupt the process (Mn2+) initial results had a greater risk for error than those we get now. As a result, I have one more thing to account for when to research this week in order to ensure that my data is meaningful. (Image is of 1 BK Virus)
Monday, February 22, 2016
Introduction to My Project
If you were to ask a random person what they thought is the most transplanted organ the answer would most likely be heart, liver, or lung. An organ that is often overlooked in terms of both importance and necessity is the kidney. These bean shaped organs are responsible for filtering and regulating the otherwise toxic cocktail of chemicals that swirl around in our bloodstream. Without them it would be impossible to live more than a week (and it definitely wouldn’t be a nice week). Unfortunately, the kidneys are also as delicate as they are important and thus are easily harmed by the effects of poor diet, obesity, diabetes, and high blood pressure, all of which have been on the rise in recent years. Consequently, the need for kidneys is more than double that of the next most needed organ (the liver) with 90,000 (2015 statistics) people on the national waiting list. It is important, therefore, to make sure that the relatively few people who can get a kidney transplant keep it functioning well for as long as possible. It is here where the BK Virus, the topic of my research makes its appearance.
A member of the polyomavirus family, the BK virus is found latent in anywhere from 80 to 90% of the population (persisting throughout the host’s entire life). Though most people remain relatively asymptomatic, this changes once they are put on a regimen of immunosuppressants. Immunosuppressants are necessary for nearly all transplant patients to ensure that the body’s natural immune response does not contribute to the destruction of the graft. The lack of antigen control allows the virus to proliferate within the graft and about 30 to 60% of those diagnosed will lose their kidney 1 year after the initial diagnosis.
In my research I will look at the incidence of BK Viremia over the past 30 years, but more than that I hope to identify a problem. With the many advances in virus and bacteria control that have occurred in the past decades such as stronger antibiotics and immunosuppressants, it's possible that we may, counterintuitively, see a rise in the number of viral infections such as these. Though we may have become more proficient in stopping our own body from attacking the transplanted organs, we may have also inadvertently opened the door for viruses that thrive in that immune response free environment to attack the very thing we’ve been trying to protect.
Throughout my internship I hope to explore this phenomenon while at the same time keeping you guys informed on both my findings and the incredible structure that is the kidney and the delicate balance within which it lives.
Subscribe to:
Posts (Atom)